Clinical research has evolved significantly in recent years, with innovative trial designs transforming the traditional paradigm. These modern approaches expedite drug development and prioritise patient-centred research.In this article, we will explore various innovative trial designs, real-world applications, and their impact on patient recruitment. While these innovations offer benefits like accelerated drug development and improved patientcentricity, they come with challenges, including regulatory complexity and data security. Additionally, platforms like Find Me Cure are bridging the gap between patients and researchers, enhancing access and diversifying the participant pool.
Clinical research has always been at the forefront of medical advancements, but the methods for conducting trials have undergone significant transformations in recent years. Innovative trial designs have emerged as a powerful force, revolutionising the way we approach clinical studies. These modern approaches not only expedite the drug development process but also ensure that patients are at the centre of the research. In this article, we will explore various innovative trial designs, their advantages, challenges, and real-world applications, shedding some light on the dynamic landscape of clinical research.
Clinical trials have historically followed a fixed and sequential structure. In such trials, researchers define a study protocol and then enrol participants following those strict criteria. This rigid design often makes it challenging to adapt to new information, resulting in inefficiencies, wasted resources, and delays in bringing effective treatments to patients.
In recent past, the landscape of clinical trials looked significantly different. Paper Case Report Forms (CRFs) were the norm, with research data painstakingly recorded by hand. This paper-based approach, while reliable, was not without its challenges. It often resulted in cumbersome data entry, transcription errors, and delays in data collection and analysis. Furthermore, monitoring the progress of trials involved on-site visits by the Clinical Research Associates (CRAs), which could be resource-intensive and time-consuming for all parties involved. Whoever hasn't transported a pile of "data collected" CRFs in their car trunk may not understand the monitoring era I'm referring to. Data management was also a labour-intensive process, often requiring meticulous attention to detail and multiple rounds of data reconciliation.
Innovative trial designs encompass a range of methodologies that break away from the traditional mould. These approaches embrace flexibility, adaptability, and efficiency, allowing researchers to learn from each patient's experience, update trial parameters, and optimiseo the research process.
The shift to innovative trial designs represents a departure from these traditional practices, embracing electronic data capture, real-time monitoring, and adaptive strategies that streamline the research process, enhance data quality, and bring us closer to the goal of more patient-centric and efficient clinical trials.
1. Adaptive Trials
Adaptive trials are designed to evolve in real-time based on the data collected. They allow for modifications to key aspects of the trial, such as the sample size, randomisation ratios, or even the treatment arms, as new information becomes available. This adaptive approach enables researchers to make informed decisions and refine the trial as it progresses.
2**. Umbrella Trials**
Umbrella trials, on the other hand, focus on a single disease or condition but test multiple treatments within that umbrella. This design is particularly effective when dealing with diseases that are highly heterogeneous, enabling researchers to identify the most suitable treatment for individual patients.
3**. Basket Trials**
Basket trials focus on specific genetic mutations or biomarkers rather than the location of the tumour. This approach allows researchers to test multiple treatments simultaneously on patients with different cancer types but the same genetic mutation. Basket trials provide a more patient-centred approach, as they match treatment to the genetic makeup of the patient.
4. Platform Trials
Platform trials are master protocols designed to investigate multiple treatments for a specific disease. They have the advantage of continuously enrolling patients and testing multiple treatments concurrently. As one treatment arm concludes, a new one can begin, ensuring that the trial remains dynamic and efficient.
The impact of innovative trial designs is best understood through real-world applications. Several notable examples have showcased the power of these modern methodologies. Each of these trials aims to address specific medical challenges, introduces innovative approaches, and offers unique benefits, along with their own set of challenges:
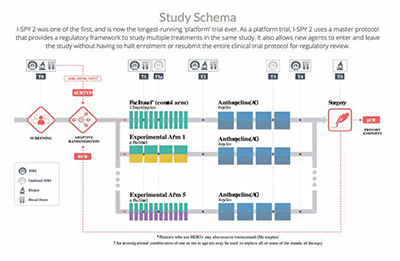
1. The I-SPY 2 Trial
ClinicalTrials.govIdentifier: NCT01042379
See Figure: 1
Aims: The I-SPY 2 trial, designed for breast cancer, aims to accelerate the development of effective treatments for breast cancer by evaluating multiple treatment regimens in the neoadjuvant setting. The primary goal is to identify and advance treatments that demonstrate the most promise.
Innovation: The trial's adaptability is its hallmark innovation. It allows for realtime assessment of treatment efficacy, enabling the removal of less effective treatments and the introduction of promising ones as the trial progresses. This approach reduces the time required to bring effective treatments to patients.
Benefits: Patients receive potentially more effective treatments in a timelier manner. The trial optimises resource utilisation by minimising the need for lengthy, separate trials for each treatment, and it accelerates decision-making for drug development.
Challenges: The adaptability of the trial necessitates rigorous statistical methods to account for changing treatment arms. Ensuring informed consent and ethical considerations regarding changes in treatment plans are ongoing challenges.
2. The National Lung Matrix Trial
ClinicalTrials.gov Identifier: NCT02664935
Aims: This umbrella trial for non-small cell lung cancer aims to match patients to targeted therapies based on specific genetic mutations. It personalizes treatment by identifying and offering treatments tailored to each patient's unique genetic profile.
Innovation: The trial's innovation lies in its personalised medicine approach, which maximises the likelihood of therapeutic benefit for patients. It shifts the focus from one-size-fits-all treatments to precision medicine.
Benefits: Patients benefit from treatments that are more likely to be effective for their specific genetic makeup. The trial minimises exposure to ineffective treatments, reducing potential side effects and optimising resource allocation.
Challenges: Identifying and enrolling patients with specific genetic mutations can be challenging. Coordinating a trial with multiple targeted treatment arms can be complex.
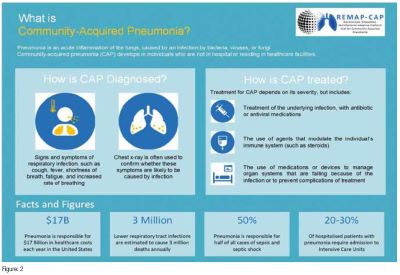
3. The REMAP-CAP Trial
ClinicalTrials.gov Identifier: NCT02735707
See Figure: 2
Aims: During the COVID-19 pandemic, the REMAP-CAP trial aimed to improve outcomes for critically ill COVID-19 patients. The trial's adaptability allowed it to quickly test multiple treatments, including novel therapies, as the pandemic evolved.
Innovation: The trial's innovation lies in its rapid adaptation to the evolving needs of the pandemic. It provides a platform for testing a wide range of treatments simultaneously.
Benefits: The trial helps identify effective treatments and improves the chances of survival and recovery for critically ill COVID-19 patients. It reduces the time needed to assess the effectiveness of potential treatments.
Challenges: Adapting the trial to a rapidly changing situation requires swift decision- making and coordination. The large amount of data generated and the need for real-time analysis can be challenging.
4. The NEJM Catalyst Innovations in Care Delivery
Aims: The NEJM Catalyst Innovations in Care Delivery initiative aims to showcase innovative trial designs and their impact on healthcare delivery and patient outcomes. It serves as a platform for sharing and disseminating insights into new approaches to care.
Innovation: The initiative itself is innovative in that it regularly highlights novel approaches and methodologies that enhance patient care delivery. It acts as a bridge for sharing best practices and innovative strategies.
Benefits: The initiative benefits healthcare professionals by providing a platform for learning about and adopting innovative approaches to care delivery. It contributes to the dissemination of best practices and innovations in the field.
Challenges: While the initiative doesn't conduct clinical trials itself, it faces challenges related to the evaluation and validation of the approaches it showcases. Ensuring that featured innovations are evidence-based and effective is crucial.
These real-world applications of innovative trial designs illustrate the diverse aims and innovations that can be achieved through modern clinical research methodologies. While they offer significant benefits, they also come with unique challenges, underscoring the need for ongoing adaptation and innovation in the field of clinical trials.
The adoption of innovative trial designs offers a multitude of benefits, transforming the clinical research landscape:
1. Accelerated Drug Development
Innovative trial designs enable faster decision-making by allowing for adaptive changes. This acceleration of the drug development process is critical, especially in the context of emerging diseases or urgent medical needs.
2. Resource Optimisation
Traditional trials often suffer from resource waste due to inefficiencies. Innovative designs allow for efficient resource allocation, reducing the overall cost of research.
3. Enhanced Patient-Centricity
Innovative trials prioritise patient needs by matching treatments with genetic profiles or adapting to patient experiences. This patient-centric approach contributes to better outcomes and improved patient satisfaction.
A great example of patient-centred approach are the Decentralised Clinical Trials. DCTs are a modern approach to clinical research that leverages technology to conduct trials with minimal reliance on physical sites, such as traditional hospitals or clinics. These trials aim to bring the research directly to patients, often in the comfort of their homes. There are some undeniable key benefits of DCTs, particularly in the context of a patient-centred approach, such as DCTs prioritiseprioritise patient convenience and comfort by allowing participants to engage from their homes, reducing the need for frequent site visits. DCTs also improve access and inclusivity by removing geographical barriers, enabling individuals in remote areas or with limited mobility to participate. They attract a more diverse pool of participants, leading to a better understanding of treatment effectiveness in different demographics. Patients in DCTs experience reduced travel, shorter wait times, and a flexible schedule, which is especially beneficial for those with chronic illnesses or ongoing treatment. Real-time data collection through remote monitoring enables researchers to access timely information on participants' health, treatment adherence, and potential side effects. DCTs also optimise resource utilisation by requiring fewer physical infrastructure and staff, resulting in cost savings and more efficient trial execution.

Despite all the benefits, there are still some challenges that need to be addressed in order to make the modern conduct of clinical research even more efficient. These challenges include ensuring data security and privacy in a decentralised environment, addressing technology access and literacy disparities, navigating regulatory hurdles, ensuring the quality of data collected remotely, maintaining participant engagement and adherence to trial protocols, addressing ethical considerations in decentralised trials, and implementing robust risk management strategies. As the field of clinical research continues to evolve, overcoming these challenges is essential to fully leverage the potential of DCTs.
4. Improved Data Quality
Real-time adaptability allows researchers to refine trial parameters, resulting in more precise and valuable data.
While innovative trial designs offer many advantages, they also come with their own set of challenges and considerations:
1. Regulatory Complexity
Regulatory bodies are still adapting to these new trial designs, which can create hurdles in approvals and compliance.
2. Statistical Rigour
Innovative designs require robust statistical methodologies to account for adaptations and multiple treatment arms.
3. Data Sharing
As these designs involve larger and more complex datasets, data sharing and transparency become increasingly important.
4. Ethical Considerations
Patient consent and the potential for changes in treatment arms pose ethical considerations that must be addressed.
A significant step towards fostering innovation in clinical research is the recognition by regulatory bodies, such as the FDA, that in some cases, animal testing may no longer be necessary. This paradigm shift acknowledges the ethical concerns surrounding animal testing and the advancement of alternative methodologies, such as in vitro testing and computational modelling. By reducing the reliance on animal models, clinical research can become more ethical, efficient, and patient-centric. This shift not only accelerates the drug development process but also aligns with the principles of innovative trial designs by prioritising the welfare of both patients and animals. It represents a promising future where scientific progress and ethical considerations can coexist harmoniously in the pursuit of medical advancements.
In the dynamic realm of clinical research, ELEM Biotech S.L. is pushing the boundaries of innovation with a groundbreaking approach that introduces virtual humans into the world of clinical trials. Their innovative methodology leverages predictive modelling and simulation, powered by supercomputers in the cloud, to create virtual populations that replicate human cells, tissues, and organs, mirroring the diversity and pathologies of society. This revolutionary technology is designed to address the entire spectrum of product development, from discovery to preclinical and clinical phases.
The integration of virtual humans offers a plethora of benefits to the clinical research landscape. It accelerates the drug development process, enhances resource efficiency, and prioritises a patient-centric approach. These virtual human models showcase how organs function in diseased states and predict the efficacy of treatments in restoring vital functions. They serve as surrogates in virtual patient trials, assist clinicians in making informed decisions, and contribute to the development of innovative treatments. ELEM's technology is designed to tackle complex physiological systems, including the cardiovascular and respiratory systems.
While the prospect of virtual humans in clinical trials is immensely promising, it comes with its set of challenges and considerations. Regulatory bodies are adapting to these new methodologies, and the integration of predictive modelling into clinical trial approval processes can be complex. Robust validation of these virtual models is essential to ensure their accuracy and reliability. Ethical considerations regarding the use of virtual humans, especially in scenarios involving the prediction of treatment outcomes, must be addressed. Additionally, it's crucial to maintain transparency and data sharing standards in the application of this technology.
In the ever-evolving landscape of clinical research, the integration of innovative trial designs represents a significant step toward revolutionising the field of clinical trials. These modern approaches, including adaptive trials, platform trials, and other innovative methodologies, collectively drive a transformation in the way clinical research is conducted.
By reducing reliance on animal testing and introducing advanced methodologies that prioritise patient welfare and resource efficiency, these innovative trial designs accelerate decision-making and bring a more ethical, efficient, and patient-centric approach to the development of medical treatments.
This pioneering work showcases how the convergence of advanced technology, predictive modelling, and clinical research can reshape the field of clinical trials. While challenges exist, the benefits are clear – a more efficient, ethical, and patient-focused approach to bringing innovative medical treatments to the world. The path to revolutionising clinical trials is now not only patientcentric but also highly technologically driven, offering a promising future for the healthcare industry.
Innovative trial designs have introduced a paradigm shift in the way clinical trials are conducted, including their impact on patient recruitment. These novel approaches have transformed the traditional recruitment landscape, ushering in significant changes, benefits, and challenges.
Thus, this may not be entirely applicable for all countries. For example, in Bulgaria or eastern Europe in general, many strategies for online patient recruitment, digitalisation, or marketing cannot be implemented. This is simply because there is still a very big social stigma, born out of common misconceptions and distrust in the pharmaceutical industry and clinical trials.
Moreover, the lack of awareness and education about the benefits of participating in clinical trials further hinders the implementation of these strategies. Without proper understanding and knowledge, potential participants may hesitate or refuse to engage in these activities.
In addition, the limited access to technology and internet infrastructure in certain regions also poses a challenge for the successful execution of online strategies. Without reliable internet connectivity and access to digital platforms, reaching out to potential patients and conducting effective marketing campaigns becomes increasingly difficult.
Therefore, it is crucial for stakeholders in these regions to address these barriers and work towards creating a more supportive environment for online patient recruitment, digitalisation, and marketing efforts. This can be achieved through targeted awareness campaigns, education initiatives, and building trust within the community. Doctors can serve as a bridge in this process, as patients trust and highly regard them as key opinion leaders. Therefore, the role of physicians in this process is crucial. By involving doctors, the potential for successful implementation of these strategies can be increased, resulting in improved clinical trial participation and overall improved quality of life via patient education.
1. Greater Access and Inclusivity:
Innovative trial designs have broadened access to clinical trials. Patients who were previously excluded due to geographic barriers, limited mobility, or other constraints now have the opportunity to participate.
2. Enhanced Patient-Centric Approach:
These designs prioritise the patient experience, making trials more convenient and comfortable. As a result, patient recruitment and retention rates have improved.
3. Improved Diversity:
Innovative trial designs often attract a more diverse participant pool, ensuring that research outcomes are more representative of real-world populations. This diversity enhances the generalisability of trial results.
4. Accelerated Recruitment:
With more patient-friendly protocols and adaptive designs, recruitment timelines are shortened. Real-time data collection enables swift identification of eligible participants.
1. Digitalisation:
Innovative trial designs utilise digital platforms for recruitment, significantly transforming the recruitment landscape. Online platforms, social media, and mobile apps have emerged as crucial channels for patient engagement. However, these methods are only effective for specific target groups, and the elderly population often faces challenges in using them. Consequently, this limits the potential indications for the particular disease being studied in the clinical trial, as these approaches are primarily applicable to younger individuals.
2. Patient Empowerment:
Patients are now better informed and empowered to seek out clinical trial opportunities independently, reducing their reliance on healthcare providers.
1. Efficient Recruitment:
Faster patient recruitment results in shorter trial timelines, reducing costs and enabling quicker access to potentially life-saving treatments.
2. Increased Engagement:
The patient-centric approach enhances engagement and willingness to participate, leading to higher retention rates.
3. Real-Time Data:
Real-time data collection and remote monitoring provide a wealth of information, helping identify eligible patients more quickly.
4. Greater Diversity:
A more diverse participant pool improves the applicability of trial results to a broader population.
1. Digital Divide:
Not all patients have equal access to the digital platforms used for recruitment, leading to disparities in trial participation.
2. Data Security:
The digitalisation of patient recruitment requires robust data security and privacy measures.
3. Regulatory Compliance:
Complying with evolving regulations, especially in the context of decentralised trials and remote recruitment, can be complex.
4. Ethical Considerations:
Ensuring informed consent and ethical conduct in digital recruitment channels is essential.
Here are some good examples of changes that emerged in patient recruitment brought about by innovative trial designs and digitalisation:
1. Online Patient Portals: Many clinical trials now offer online patient portals that allow potential participants to easily access trial information, complete eligibility questionnaires, and express their interest in joining a trial. Patients can browse trial details, eligibility criteria, and frequently asked questions, all from the comfort of their homes.
Find Me Cure is a Bulgarian startup, which created a platform dedicated to connecting patients with clinical trials. It serves as a bridge between patients and researchers, helping potential participants find suitable trials while assisting researchers in locating eligible candidates.
Find Me Cure plays a significant role in transforming the landscape of clinical trials by enhancing access to clinical trial information, improving patient engagement, streamlining the recruitment process for researchers, and contributing to diversifying the participant pool.
Find Me Cure is bridging the gap between potential participants and researchers. By providing easy access to clinical trial information, Find Me Cure empowers patients to proactively seek trials that match their specific conditions and preferences. This enhanced access enables patients to take a more proactive role in their healthcare journey, empowering them with the knowledge and resources to make informed decisions about their treatment options.
Moreover, Find Me Cure contributes to improved patient engagement in clinical trials. The platform helps patients navigate the often complex landscape of clinical trials, providing them with the necessary information and resources to understand the trial process and requirements. This increased engagement not only leads to higher participation rates but also ensures that patients are actively involved throughout the trial, ultimately improving the quality and reliability of the research outcomes.
In addition, Find Me Cure streamlines the recruitment process for researchers. By providing a centralised platform for researchers to identify and engage with potential participants, the platform simplifies the often time-consuming and resource-intensive process of finding eligible candidates for clinical trials. This efficiency in recruitment allows researchers to quickly and effectively identify suitable participants, accelerating the overall trial timeline and facilitating faster access to potentially life-saving treatments.
1. Social Media Campaigns:
Researchers and organisations use social media platforms like Facebook, Twitter, and Instagram to run targeted ad campaigns that reach a broader audience. Patients can come across trial opportunities while scrolling through their feeds, and these campaigns can provide direct links to trial websites or registration pages.
2. Mobile Apps:
Clinical trial sponsors have developed mobile apps that enable patients to search for trials, receive notifications about new studies, and complete prescreening questionnaires. These apps facilitate seamless interaction between patients and clinical trial teams.
3. Email and SMS Alerts:
Patients can subscribe to receive email or SMS alerts about clinical trial opportunities. These alerts notify them of new trials that match their profiles and preferences, reducing the need to actively seek out trials.
4. Virtual Recruitment Events:
Virtual events, including webinars and information sessions, have become popular for recruiting patients. These events allow potential participants to learn about the trial, ask questions, and express their interest, all without the need to attend in-person meetings.
5. Online Patient Communities:
Online communities and forums dedicated to specific medical conditions or diseases have emerged as valuable recruitment tools. Researchers can engage with these communities to inform patients about relevant trials and provide study details.
6. Remote Screening and Telemedicine:
Digital recruitment goes beyond just finding trials. Remote screening and telemedicine enable initial assessments, eligibility checks, and consultations to occur online, minimising the need for patients to travel to physical sites for these processes.
7. Web-Based Pre-Screening Tools:
Many trials offer web-based pre-screening tools that allow patients to quickly check their eligibility by answering a series of questions. If they meet the criteria, they can express their interest in participating.
8. Patient Advocacy Groups:
Advocacy groups often collaborate with researchers to promote trial opportunities within their networks. This approach is especially effective in recruiting patients who are already engaged with the advocacy group's activities.
9. Patient Registries:
Many diseases and conditions have patient registries that collect and store information about individuals interested in participating in research. These registries are valuable resources for identifying potential trial participants.
These changes in patient recruitment reflect the shift toward a more patientcentric and digitalised approach. They make it easier for patients to access trial information, express interest, and actively engage with the clinical trial process. However, they also bring challenges related to data privacy, digital literacy, and the need for clear and ethical communication throughout the recruitment process.
Innovative trial designs have transformed patient recruitment by increasing inclusivity, enhancing patient-centric approaches, and accelerating recruitment timelines. However, digitalisation, data security, regulatory compliance, and ethical considerations pose challenges. Platforms like Find Me Cure play a crucial role in making clinical trials more accessible and efficient, ultimately benefiting both patients and researchers.
In light of the numerous benefits and despite the inherent challenges and areas that require improvement, I is my belief that innovation is not only welcome, but a crucial necessity for the advancement of medicine. The landscape of clinical trials has evolved significantly, thanks to innovative trial designs, which offer the potential for accelerated drug development, enhanced patient-centricity, and improved data quality.
While it's true that these innovations come with their set of issues, it's essential to emphasise that the pharmaceutical and clinical trial industry is deeply committed to safety and rigour. Stringent testing, adherence to multiple guidelines, and rigorous oversight are intrinsic to this field. Mistakes are highly consequential, and as a result, ensuring the safety of participants is paramount. It's a testament to the industry's dedication to patient welfare that, despite the complexity of clinical trials, adverse events are exceedingly rare. These innovations not only drive the medical field forward but also align with the broader goals of improving patient care and advancing healthcare outcomes.